Because carbon atoms can arrange themselves in several distinct ways, materials made from only carbon can take on several different forms (or allotropes). Two familiar forms of pure carbon are graphite and diamond. However, scientists have more recently discovered new forms of pure carbon such as fullerenes (or buckyballs) and carbon nanotubes (CNTs). Each allotrope has dramatically different properties.
|
Graphite |
||
|
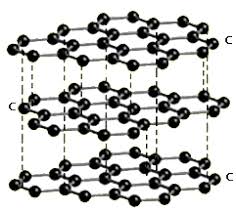
Graphite is very soft and slippery. For this reason, it is used in pencils and as a lubricant in industrial machinery. Graphite’s softness and slipperiness arises from the way the carbon atoms are arranged on the nanoscale. Graphite consists of sheets of carbon atoms. In these sheets, the carbon atoms are arranged in a hexagonal, or honey comb. Each carbon atom is strongly bonded to its three neighboring carbon atoms. These hexagonal sheets then appear to stack upon each other to create the overall structure of graphite. The bonding between the sheets is much weaker than the bonding between the atoms in the planes. Therefore, when a small force is applied to the graphite structure (e.g. dragging a pencil across a sheet of paper) the sheets of carbon slip off a few layers at a time. Graphite conducts electricity. The electrode in a common dry cell battery is made of graphite. |
 |
|
|
Diamond |
||
|
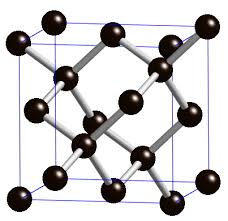
Diamonds are the hardest material found in nature. A diamond ring can scratch glass easily, and the teeth of some saw blades contain tiny diamonds to help them better cut through concrete, bricks and wood. Diamond’s hardness (and its other properties) comes from its nanostructure—the way that the carbon atoms are arranged on the nanoscale. Each carbon atoms is strongly bonded to four neighboring carbon atoms, in a three-dimensional arrangement. This 3-dimensional arrangement, called a tetrahedron, (along with the strength of the bonds between the carbons) makes the structure very rigid and hard. |
 |
|
|
|
||
|
Buckminsterfullerenes (also known as buckyballs) |
||
|
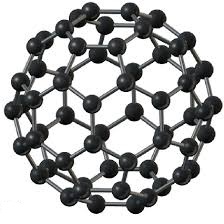
A buckminsterfullerene molecule, also known as a fullerene or “buckyball,” contains 60 carbon atoms arranged to form a hollow sphere. Its molecular formula is C60. Each carbon atom in the sphere is bonded to its three neighboring carbons. The atoms are arranged to form a mix hexagons and pentagons. This same pattern of hexagons and pentagons can be seen when looking at a soccer ball. The buckminsterfullerene molecule was named after an architect, Robert Buckminster Fuller. The buckyball was first discovered in 1985, by Richard Smalley, Robert Curl and Harold Kroto. They discovered it during an experiment where a laser heated graphite until it evaporated. The remaining soot contained a small fraction of buckyballs. In 1996 Smalley, Curl and Kroto won the Nobel Prize in Chemistry for their discovery. |
 |
|
|
Carbon nanotubes (CNTs) |
||
|
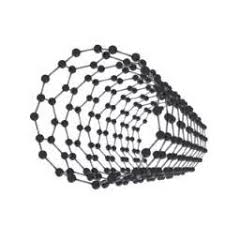
Carbon nanotubes (CNTs) are a more recently discovered form of carbon. It is convenient to think of a CNT as a sheet of graphite (also called a graphene sheet) rolled up to form a tube. However, this is NOT how CNTs are formed. CNTs were discovered accidently by Sujimo Iijima in 1991. While making buckyballs, Iijima noticed long, needlelike structures (the CNTs) mixed in with the buckyballs. CNTs are also classified as single-walled (SWNT) or multi-walled (MWNT). SWNTs resemble a single graphene sheet rolled up, while MWNT are made up of several SWNTs nested inside each other. |
 |
|